Table of Contents
ToggleHeat and Thermodynamics
Heat and thermodynamics are branch of physics that studies the relationship between heat, work, temperature, and energy, and the physical laws that govern the behavior of these quantities in systems.
Heat Engine
Heat engine is a device used for converting heat energy into mechanical energy is called a heat engine. The main parts of the heat engine are:
a) Source:
It is a hot body (reservoir) at a constant high temperature from which the heat engine can draw any amount of heat. The characteristic of the source is that whatever heat is taken out from it, its temperature will not decrease. For example, ocean, sun, burning petrol engine, etc. are the hot reservoir (source).
b) Sink:
It is a cold body at a constant low temperature to which any amount of heat can be rejected. The characteristic of the sink is that whatever heat is given to it, its temperature will not rise. For example, ocean water, atmosphere is a sink.
c) Working substance:
The working substance is an ideal gas. It takes heat from the source and converts a part of absorbed heat into mechanical work. The remaining heat is rejected to the sink.
Efficiency of Heat Engine
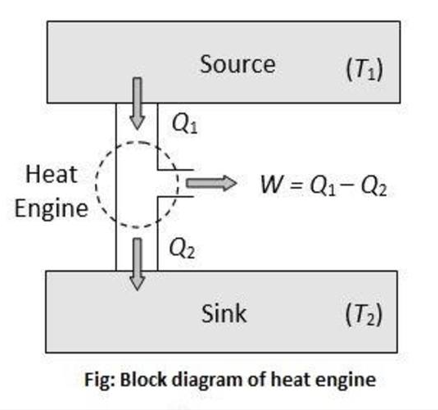
Let Q1 be the amount of heat absorbed by the working substance from the source at a higher temperatureT1, W be the mechanical work done by the working substance, and Q2 be the remaining part of the heat rejected to the sink at a lower temperature T2
∴W=Q1−Q2
The performance of a heat engine is expressed by means of efficiency (η), which is defined as the ratio of useful work obtained to the heat energy supplied to it from the source.
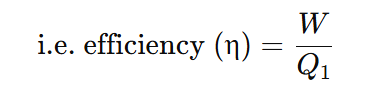
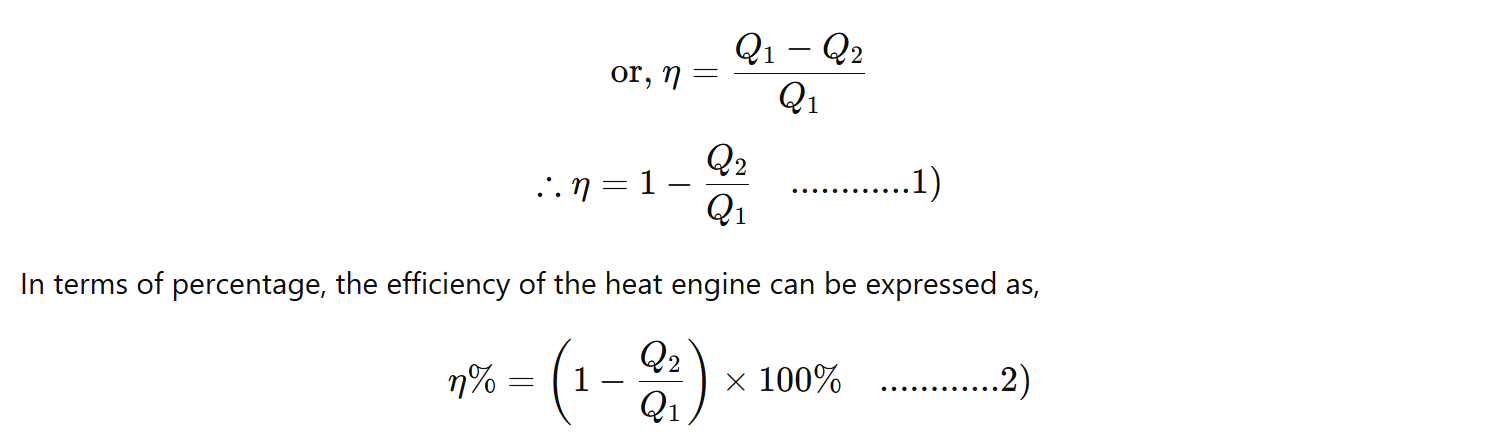
NOTE:
The efficiency of the engine can never be 1 (or 100%) because the ratio Q2\Q1 can never be zero. For the heat engine to have an efficiency of 100%, Q2 should be zero, which means all the heat energy absorbed from the source is converted to work and no amount of heat is rejected to the sink. And it is not possible to design a machine if there is no heat rejected to the sink, because if no heat is rejected to the sink then there is no flow of heat, and work will not be done. Our first requirement is that there must be a flow of heat for the work to be done. And for that, certain heat must be rejected to the sink. So has a certain value. Hence, Q2\Q1 is never zero.
Therefore, the efficiency of a machine is never 1, i.e., 100%. It is always less than 1 (i.e., no heat engine is 100% efficient).
Moreover, the efficiency of a heat engine can be expressed in terms of the temperature of the source (T1) and the temperature of the sink (T2) as,
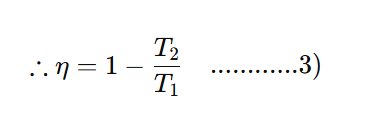
Second law of thermodynamics:
The second law of thermodynamics can be stated in two ways:
a) Kelvin–Planck’s statement:
“It is impossible to design an engine that extracts heat and fully utilises into work without producing any other effect.”
From this statement it is clear that any amount of heat can never be converted completely into work. It is essential for an engine to reject some amount of heat to the sink. An engine essentially requires a source as well as sink. The efficiency of an engine is always less than unity because heat cannot be fully converted into work.
b) Clausius statement:
“It is Impossible to make heat flow from a colder body to a warmer body without doing external work.”
From Clausius’ statement, it is clear that heat cannot flow from a body at low temperature to one at a higher temperature unless work is done by an external agency. For e.g., a body at a lower gravitational potential level cannot move up to a higher level without work done by an external agency.
NOTE: A Refrigerator is a heat engine that transfers heat from a cold body to a hot body with the help of an external agency, i.e., electricity.
Carnot Engine:
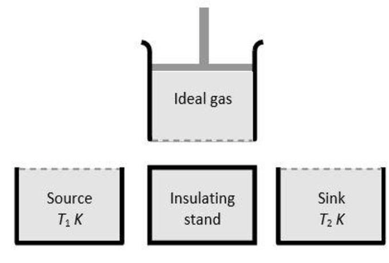
A Carnot engine is a theoretical engine that is used to convert heat into mechanical work. It is an ideal heat engine with maximum efficiency. It consists of the following parts:
i) A source of infinite thermal (heat) capacity maintained at a constant higher temperature T₁ from which the working substance draws heat.
ii) A sink of infinite thermal capacity maintained at constant lower temperature T₂ to which any amount of heat can be rejected.
iii) A cylinder with perfectly non-conducting (insulating) walls and a perfectly conducting base containing a perfect ideal gas as working substance and fitted with a non-conducting frictionless piston.
iv) A perfectly non-conducting (insulating) stand for the cylinder.
Carnot cycle :
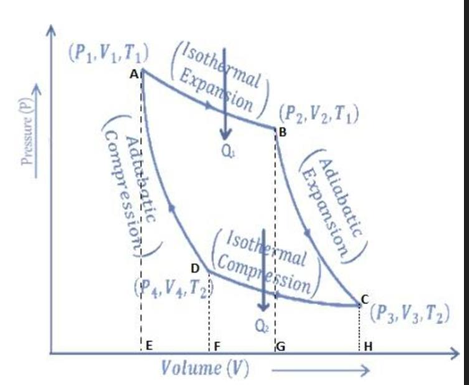
As the engine works, the working substance of the engine undergoes a cycle known as the Carnot cycle. It consists of four steps:
a) Step I (Isothermal expansion) (curve AB) :
The cylinder containing an ideal gas as the working substance is allowed to expand isothermally from initial state A (P1, V1, T1) to final state B (P2,V2,T1) at constant temperature with absorption of heat Q1. According to the first law of thermodynamics, the work done during isothermal expansion is,
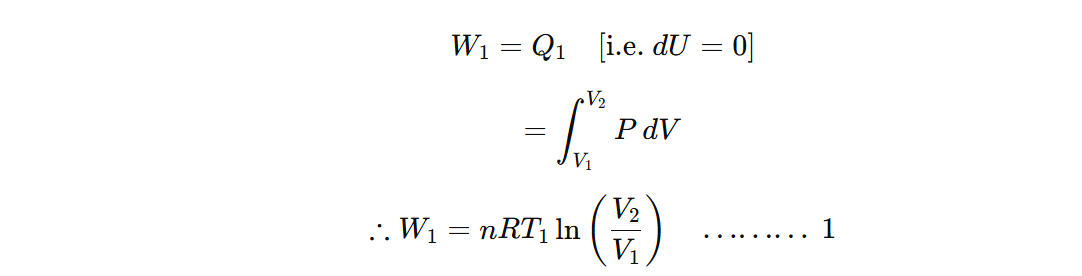
Also, W1=area of ABGEA
b) Step II (Adiabatic expansion) (curve BC) :
The cylinder is then placed on the insulating stand and the gas is allowed to expand adiabatically till the temperature falls from T1 toT2. Then the work done during adiabatic expansion is,
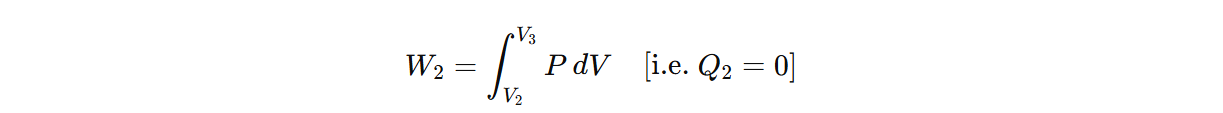
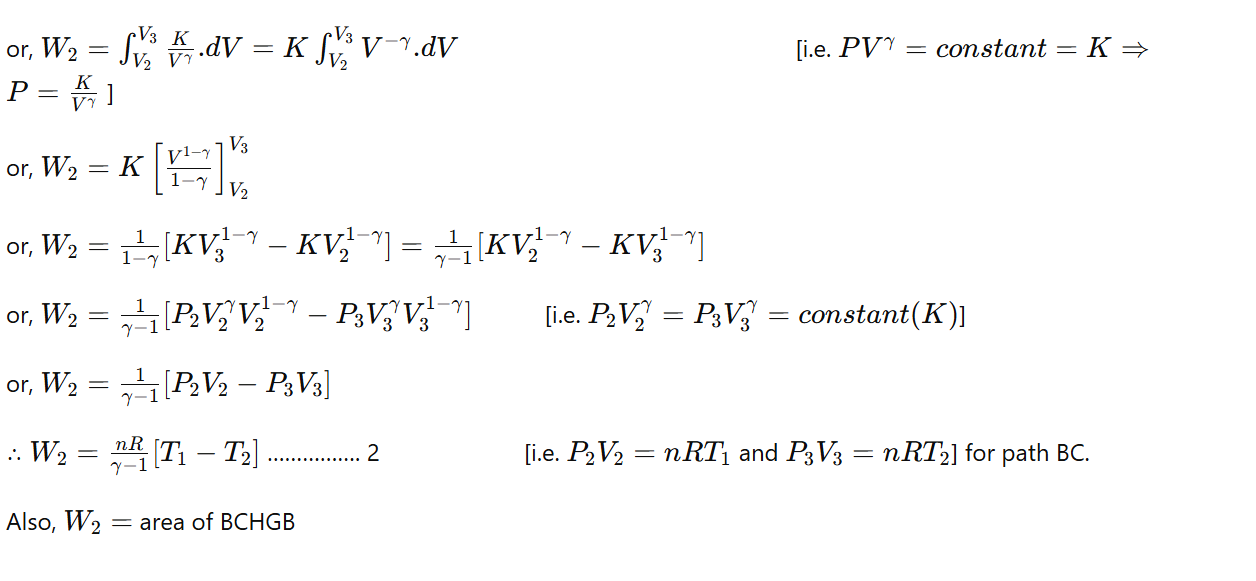
c) Step III (Isothermal compression) (curve CD):
The cylinder is placed on the sink, and the gas is compressed isothermally at constant temperature T2 with rejection of heat Q2 to the sink. Then the work done during isothermal compression is,
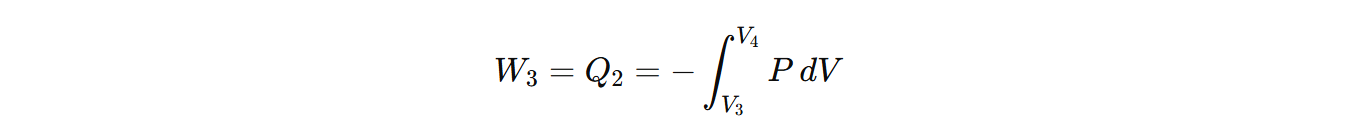
) Step IV (Adiabatic compression) (curve DA):
Finally, the cylinder is again placed on an insulating stand and the compression is continued so that the gas returns to its initial state. Then work done during adiabatic compression from sate D (P4,V4,T2) to state A (P1,V1,T1) is
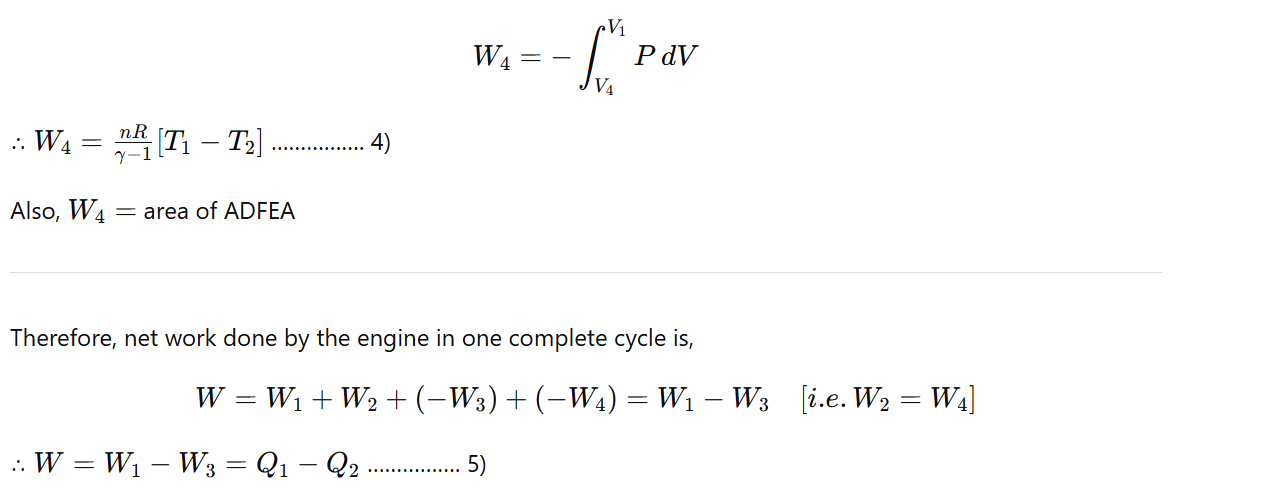
Also, W = area of ABGEA + area of BCHGB – area of CDFHC – area of ADFEA
∴ W = Area of ABCDA ……………. (6)
Hence, in the Carnot engine, the net work done by the engine per cycle is numerically equal to the area of the ABCDA representing the cycle.
Efficiency of Carnot cycle:
It is defined as the ratio of external work done by the engine (W) to the amount of heat absorbed from the source (Q₁).

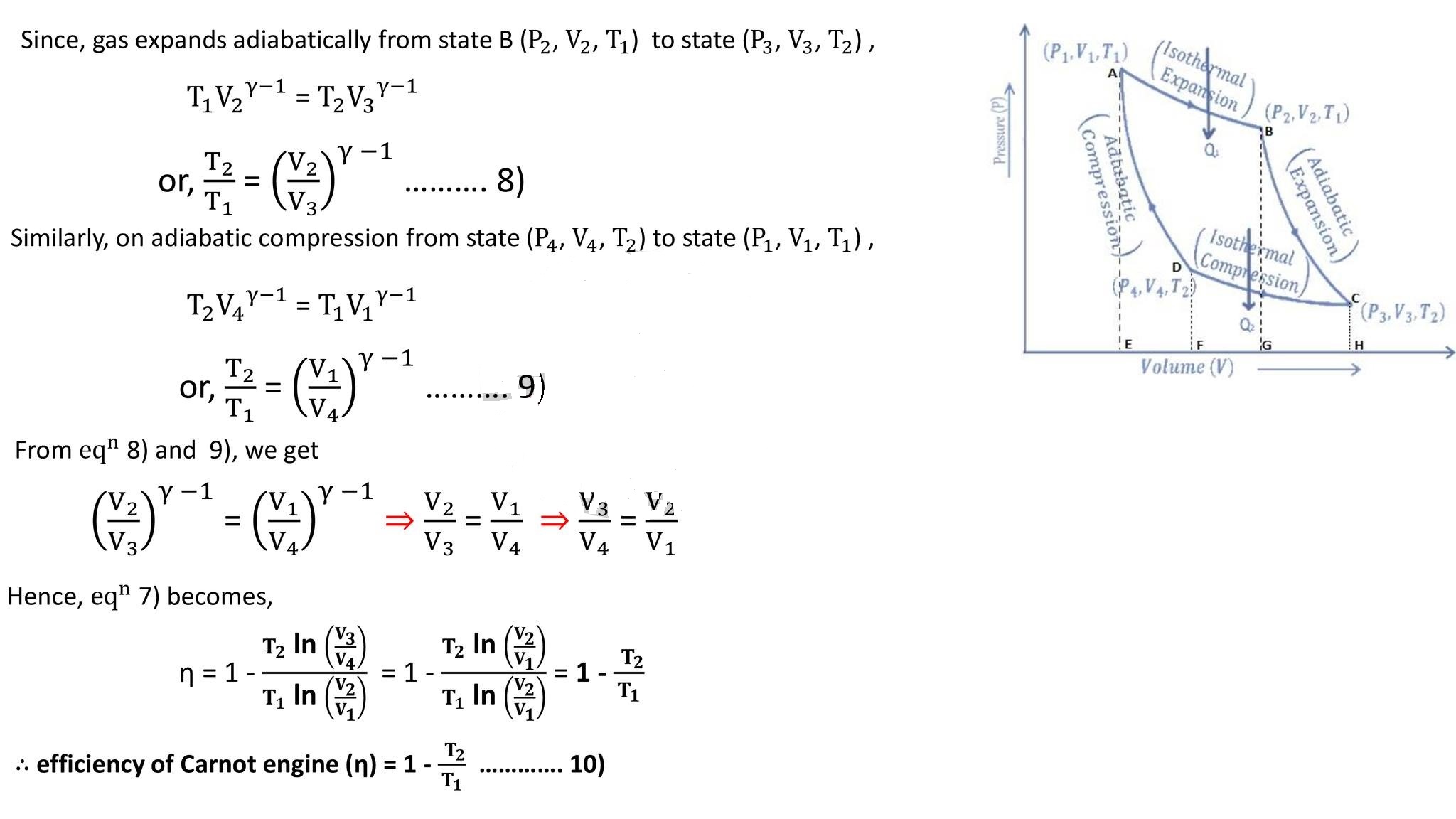
Eqn (10) shows that the efficiency of a heat engine depends only on the temperature of the source (T1) and sink (T2)(and is independent of all other factors.
⇒ As on Kelvin scale, temperature can never be negative (as 0 K is defined as the lowest possible temperature) and T1 and are finite, the efficiency of a heat engine is always less than unity, i.e., the whole of the heat can never be converted into work, which is in accordance with the second law.
Reversibility of Carnot’s Engine:
Carnot’s engine is perfectly reversible because,
→ There is no friction between the cylinder and the piston.
→ The operations on the ideal gas should be performed very slowly.
→ The loss of heat is prevented by using an insulated piston and insulated walls of the cylinder.
→ The base of the cylinder is conducting such that the temperature remains constant due to infinite thermal capacities of the source and sink (during the isothermal process).
NOTE: Carnot’s reversible engine working between two given temperatures is considered to be the most efficient engine.
Types of Heat Engine:
There are two types of heat engines:
a) External Combustion Engine: In this engine, the combustion of fuel takes place outside the engine. For example: Steam engine.
b) Internal Combustion Engine: In this engine, the combustion of fuel takes place inside the engine. For example: Petrol engine, diesel engine. These two engines are described below:
Petrol Engine (Otto Engine):
The internal combustion heat engine, which uses a mixture of air and petrol (98% air and 2% petrol vapour) as the working substance, is called a petrol engine. It consists of a cylinder fitted with a piston. The cylinder is provided with the inlet valve, exhaust valve, and sparking plug as shown in the figure. The Otto cycle consists of the following four strokes:
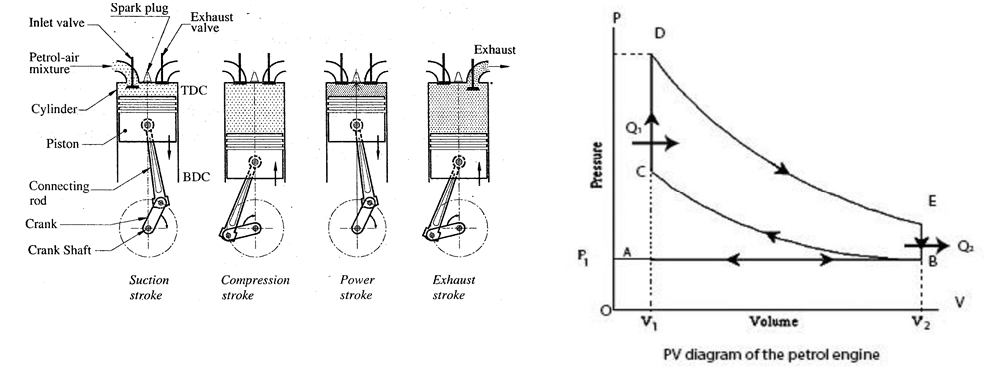
a) Suction Stroke (Intake Stroke):
In this stroke, the inlet valve opens and a suitable amount of working substance (air-petrol mixture) enters inside the cylinder due to the outward (downward) motion of the piston, which is represented by curve AB in the P–V diagram. The exhaust valve remains closed throughout the stroke.
b) Compression Stroke:
In this stroke, both the inlet and exhaust valves remain closed. The mixture undergoes adiabatic compression from B to C, so that the temperature of the mixture rises to about 600°C. After compression (just before the end of this stroke), the sparking plug produces a spark, ignition takes place, and Q₁ heat is absorbed, which is represented by CD in the indicator diagram.
c) Working Stroke (Power Stroke or Expansion Stroke):
During this stroke, both the inlet and exhaust valves remain closed. Due to the ignition of the mixture, the piston is pushed down with great force, i.e., adiabatic expansion takes place. Thus, the work is obtained in this stroke. It is represented by the DE line in the indicator diagram.
d) Exhaust stroke:
In this stroke, the exhaust valve opens, whereas the inlet valve remains closed, and burnt out gases are exhausted out and heat Q2 is rejected to the surroundings, which is represented by EB in the indicator diagram. After exhaustion, a closed cycle is completed, which is called the Otto cycle, and the cylinder is ready to start another cycle. It is represented by BA in the indicator diagram.
Efficiency of petrol Engine:
The efficiency of a petrol engine is given by
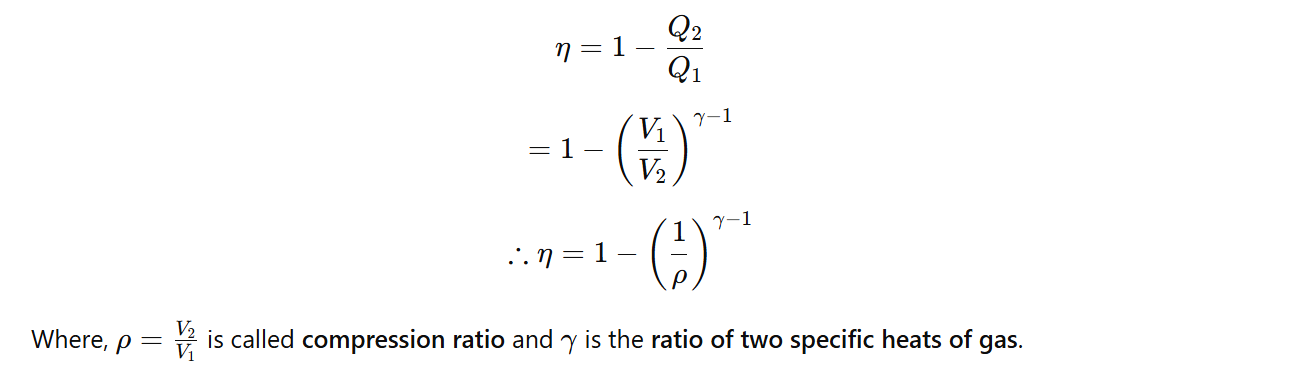
Diesel Engine
A diesel engine is an internal combustion heat engine that uses an air-diesel mixture as fuel. It consists of a cylinder fitted with a piston. The cylinder is provided with the inlet valve, exhaust valve, and oil supply valve (fuel injector) as shown in fig. The diesel cycle consists of the following four strokes.
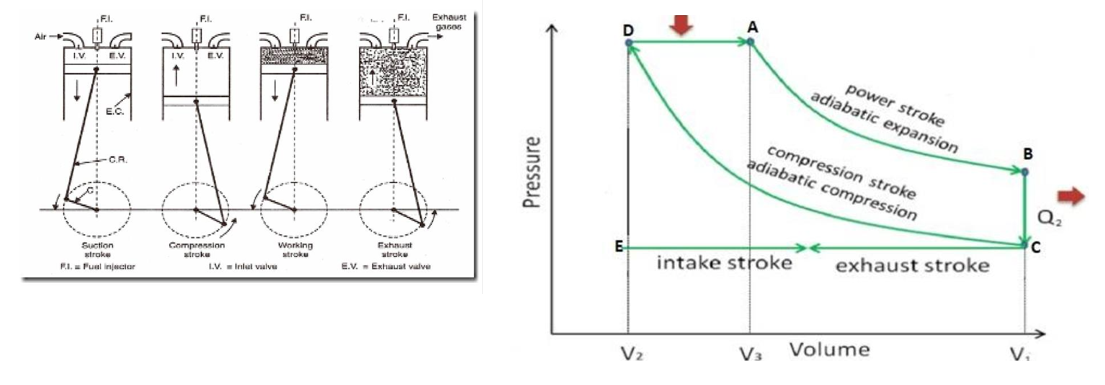
a) Suction stroke (Intake stroke):
In this stroke, the inlet valve opens and air at atmospheric pressure is drawn inside the cylinder due to downward motion of the piston, which is represented by EC in the PV-diagram. The exhaust valve remains closed throughout the stroke.
b) Compression stroke:
In this stroke, all the valves remain closed. The air inside the cylinder is compressed adiabatically to high pressure and temperature (~1000°C) by the upward motion of the piston. This compression is represented by CD in the PV-diagram.
c) Working stroke (Power stroke or Expansion stroke):
In this stroke, the quantity of fuel (diesel vapour) is injected into the hot compressed air in fine sprays by the fuel injector, and it (fuel) starts to burn instantly at constant pressure, which produces a large amount of heat, which is represented by DA in the PV-diagram. Due to this heat, pressure increases and pushes the piston downward with an adiabatic increase of volume. Thus, work is obtained which is represented by AB in the PV-diagram.
d) Exhaust stroke:
As the piston moves downwards, the exhaust valve is opened and the burnt-out gases escape to the atmosphere. Finally, the exhaust valve closes and the cycle is completed, which is called the diesel cycl,e and the engine is ready for a new cycle. This stroke is represented by CE in the indicator diagram.
Efficiency of Diseal Engine
The efficiency of a diesel engine is given by,


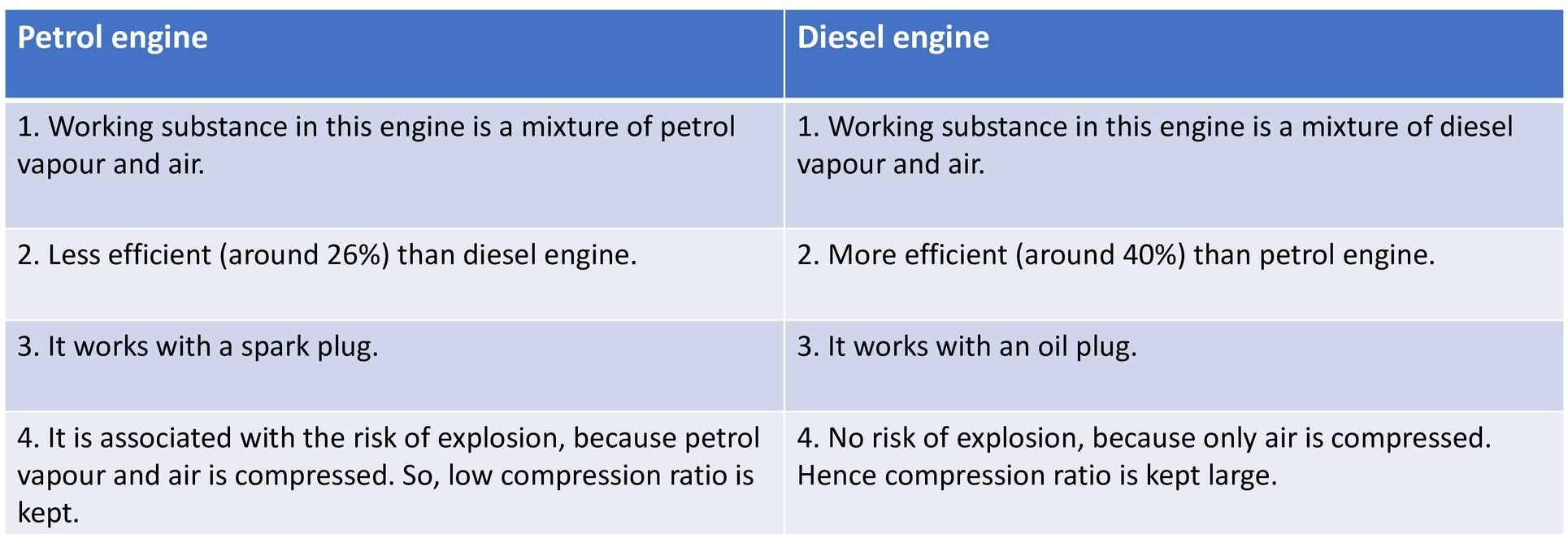
Comparison between Petrol engine and Deiseal engine
Refrigerator (Heat Pump)
A refrigerator is a heat engine that operates in the reverse direction. It essentially consists of three parts:
- Source: At higher temperature T1
- Sink: At lower temperature T2
Working substance: Refrigerant liquid ammonia
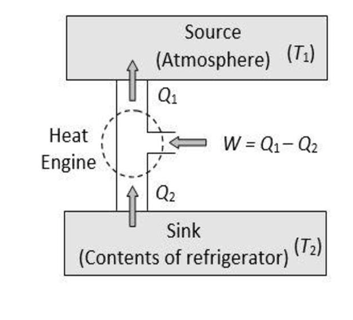
In a refrigerator, the working substance takes heat Q2 from a sink (contents of the refrigerator) at a lower temperature T2, has a net amount of work W done on it by an external agent (usually the compressor of the refrigerator), and gives out a larger amount of heat Q1 to a hot body at a temperature T1 (usually the atmosphere), i.e.
Q1=W+Q2
Thus, it transfers heat from a cold to a hot body at the expense of mechanical (electrical) energy supplied to it by an external agent. The cold body is thus cooled more and more.
The performance of a refrigerator is expressed by means of the “coefficient of performance” (β), which is defined as the ratio of the heat extracted from the cold body to the work needed to transfer it to the hot body.
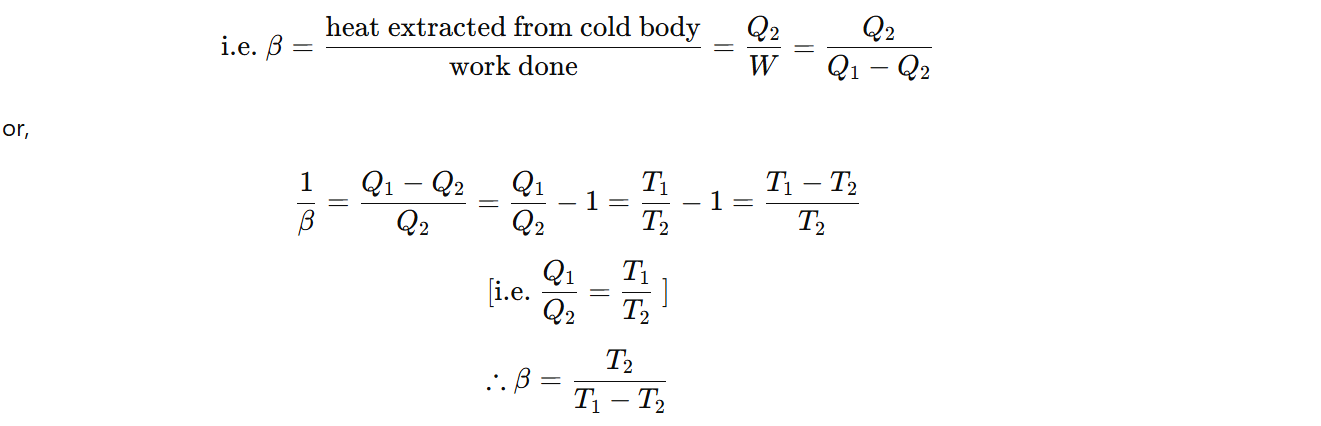
Entropy:
Entropy is a measure of the disorder of molecular motion in a system. The greater the disorder, the greater is the entropy. From the First Law of Thermodynamics,
dQ=dU+dW
Where,
- dQ = amount of heat added to the system
- dU = internal energy of the system
- dW = work done by the system
In an isothermal process, dU = 0 (i.e., temperature remains constant in this process). As internal energy is a function of temperature, there is no change in internal energy during isothermal expansion.

The gas (system) is now in a more disordered state after expansion than before, because the molecules are moving in a larger space in more random positions. The ratio (dV/V) is a measure of the increase in disorder and is proportional to the quantity dQ/T.
So, the change in entropy of a system (dS) at temperature T is,
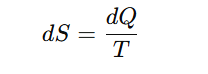
The SI unit of entropy is joules per kelvin (J/K). This relation is called the mathematical form of the Second Law of Thermodynamics.
NOTE:
Entropy remains constant during an adiabatic process, i.e., dS = 0, because there is no exchange of heat between the system and surroundings (i.e., dQ = 0).
So, there is no change in entropy.
NUMERICALS:
Q.1) An ideal heat engine operates between two reservoirs at two temperatures. In order to achieve 30% efficiency when the temperature of the sink is 50°C, what should be the temperature of the source?
[Ans: 461.43 K]
Q.2) The source reservoir of a Carnot engine is at a temperature of 400 K and takes 400 J of heat and rejects 20 J of heat to the sink reservoir in each cycle. What is the efficiency of the engine and the temperature of the sink?
[Ans: 95%, 20 K]
Q.3) A Carnot engine whose low temperature reservoir is at 27°C has an efficiency of 25%. In order to increase the efficiency to 50%, how much should the temperature of the high temperature reservoir be increased, if the temperature of the low temperature reservoir remains constant?
[Ans: 200 K]
Q.4) A Carnot engine has 50% efficiency with a sink at 9°C. By how many degrees should the temperature of the source be increased in order to raise the efficiency to 70%, keeping the sink temperature constant?
[Ans: 376 K]
Q.5) A Carnot engine works between the source and the sink with an efficiency of 40%. How much should the temperature of the sink be lowered, keeping the source temperature constant at 27°C, so that its efficiency increases by 10%?
[Ans: 30 K]
Q.6) A Carnot engine works between 800°C and 400°C. If it is possible either to increase the source temperature by 50°C or to decrease the sink temperature by 50°C, which of these actions will cause a greater increase in efficiency? Justify your answer.
[Ans: To decrease temperature of sink (42%) is more efficient than increasing source (40.1%)]
Q.7) The efficiency of a Carnot cycle is 15%. If on reducing the temperature of the sink by 65°C, the efficiency becomes 30%, find the initial and final temperatures between which the cycle is working.
[Ans: 428.3 K, 368.3 K]
Q.8) A petrol engine consumes 10 kg of petrol in one hour. If the power of the engine is 20 kW and the calorific value of petrol is 11.4 × 10³ cal/gm, calculate the efficiency of the engine.
[Ans: 15%]
Compiled by Er. Basant Kumar Yadav
